Acute Ischemic Stroke Therapeutics Market to Grow by USD 1.74 Billion from 2024-2028, Driven by Rising Cardiovascular Disease Prevalence and AI-Driven Market Transformation - Technavio
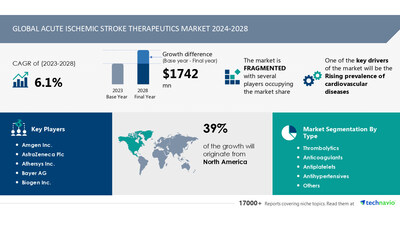
NEW YORK, Oct. 17, 2024 /PRNewswire/ -- Report with the AI impact on market trends - The Global Acute Ischemic Stroke Therapeutics Market size is estimated to grow by USD 1.74 billion from 2024-2028, according to Technavio. The market is estimated to grow at a CAGR of 6.1% during the forecast period.Rising prevalence of cardiovascular diseases is driving market growth, with a trend towards integrating genomic and clinical data through translational bioinformatics in acute ischemic stroke management. However, unmet needs for anticoagulant reversal agents poses a challenge - Key market players include Amgen Inc., AstraZeneca Plc, Athersys Inc., Bayer AG, Biogen Inc., Boehringer Ingelheim International GmbH, Bristol Myers Squibb Co., Daiichi Sankyo Co. Ltd., F. Hoffmann La Roche Ltd., Johnson and Johnson Services Inc., Medtronic PLC, Merck and Co. Inc., NoNO Inc., Pfizer Inc., SanBio Co. Ltd., Sanofi SA, Stryker Corp., and Teva Pharmaceutical Industries Ltd..
Key insights into market evolution with AI-powered analysis. Explore trends, segmentation, and growth drivers- View the snapshot of this report
Acute Ischemic Stroke Therapeutics Market Scope |
|
Report Coverage |
Details |
Base year |
2023 |
Historic period |
2018 - 2022 |
Forecast period |
2024-2028 |
Growth momentum & CAGR |
Accelerate at a CAGR of 6.1% |
Market growth 2024-2028 |
USD 1742 million |
Market structure |
Fragmented |
YoY growth 2022-2023 (%) |
5.7 |
Regional analysis |
North America, Europe, Asia, and Rest of World (ROW) |
Performing market contribution |
North America at 39% |
Key countries |
US, UK, Germany, China, and Canada |
Key companies profiled |
Amgen Inc., AstraZeneca Plc, Athersys Inc., Bayer AG, Biogen Inc., Boehringer Ingelheim International GmbH, Bristol Myers Squibb Co., Daiichi Sankyo Co. Ltd., F. Hoffmann La Roche Ltd., Johnson and Johnson Services Inc., Medtronic PLC, Merck and Co. Inc., NoNO Inc., Pfizer Inc., SanBio Co. Ltd., Sanofi SA, Stryker Corp., and Teva Pharmaceutical Industries Ltd. |
Market Driver
Acute Ischemic Stroke (AIS) is a complex cardiac disorder requiring integration of genotypic and clinical data for effective treatment and prevention. Modern approaches, such as Translational Bioinformatics (TBI), are being developed to analyze heterogeneous features from bioinformatics, biostatistics, statistical genetics, and clinical informatics. TBI acts as a data-storage source, integrating and improving data representation using tools like Ontologies and Controlled Vocabulary (CV). In AIS, genotypic data (presence or absence of risk genes like HDAC9, PITX2, ZFHX3, NINJ2, PRKCH, and 9p21) and clinical data (clinical tests like magnetic resonance imaging and patient history) are integrated. The clinical history includes age of diagnosis and family history of sudden death. Compilation of these details enhances doctors' understanding of each patient's clinical scenario, leading to improved treatment approaches. This integration of genotypic and clinical data will fuel the growth of the AIS therapeutics market during the forecast period.
The Acute Ischemic Stroke (AIS) market is witnessing significant growth due to the increasing prevalence of AIS caused by atherosclerosis plaques obstructing blood flow to the brain. Neuroimaging techniques such as Computed Tomography (CT) scans and Magnetic Resonance Imaging (MRI) scans play a crucial role in diagnosing AIS. Surgical equipment like Carotid Endarterectomy and Angioplasty are used for removing blockages in arteries, while Endovascular Mechanical Thrombectomy and clot dissolving drugs like Tissue Plasminogen Activator (tPA) and tenecteplase are used for removing blood clots. The market is driven by an aging population, rising prevalence of conditions like high blood pressure, diabetes, and obesity, and advancements in neuroimaging technologies. Key players in the market include Defense Health Agency, alteplase (Drug Class: Thrombolytics), tenecteplase (Drug Class: Thrombolytics), antiplatelets, anticoagulants, statins, antihypertensives, and other drug classes. The route of administration for these drugs can be intravenous or intracranial.
Request Sample of our comprehensive report now to stay ahead in the AI-driven market evolution!
Market Challenges
- The Acute Ischemic Stroke Therapeutics Market faces a significant challenge due to the limited availability of antidotes for anticoagulant reversal agents. While anticoagulants are essential for treating Acute Ischemic Strokes (AIS), they carry the risk of excessive bleeding, which can be reversed using reversal agents. Currently, vitamin K, protamine, and prothrombin complex concentrates are available to reverse the effects of warfarin and heparin. However, the acceptance of these drugs is low due to the scarcity of antidotes for newer anticoagulants like direct thrombin inhibitors and factor XA inhibitors. The recent approval of idarucizumab, an antidote for dabigatran, is a positive development. However, ongoing research is necessary to develop reversal agents for newer anticoagulants, which will expand the market's growth potential during the forecast period. The unmet need for anticoagulant reversal agents hinders the adoption of novel anticoagulants, negatively impacting the market's growth.
- The Acute Ischemic Stroke Therapeutics market is focused on developing innovative treatments for blood clots in the arteries supplying the brain. The primary challenges include the complexity of the brain's blood vessels and the need for rapid intervention to prevent brain cell damage. Surgical equipment like mechanical thrombectomy devices and clot dissolving drugs such as alteplase and tenecteplase are key solutions. An aging population, medical conditions like high blood pressure, diabetes, and obesity, and neuroimaging technologies like CT scans and MRI scans are significant market drivers. Defense Health Agency and other healthcare providers utilize various drug classes, including thrombolytics, antiplatelets, anticoagulants, statins, antihypertensives, and others. The distribution channel includes hospital pharmacies, retail pharmacies, and online pharmacies, with the route of administration being parenteral.
Discover how AI is revolutionizing market trends- Get your access now!
Segment Overview
This acute ischemic stroke therapeutics market report extensively covers market segmentation by
- Type
- 1.1 Thrombolytics
- 1.2 Anticoagulants
- 1.3 Antiplatelets
- 1.4 Antihypertensives
- 1.5 Others
- Distribution Channel
- 2.1 Hospital pharmacies
- 2.2 Retail pharmacies
- 2.3 Online pharmacies
- Geography
- 3.1 North America
- 3.2 Europe
- 3.3 Asia
- 3.4 Rest of World (ROW)
1.1 Thrombolytics- Thrombolytic agents, also known as clot-busting drugs, are the primary therapeutics used for treating Acute Ischemic Strokes (AIS). The process of breaking down blood clots, known as thrombolysis, is essential for restoring blood flow to the affected brain areas. The most widely used thrombolytic agent for AIS is Activase, a tissue plasminogen activator sold under the brand name by Roche. The FDA recommends a dosage of 0.9 mg per kg body weight infused intravenously over an hour, with 10% administered as an initial bolus within one minute. Activase is approved for various indications, including AIS, acute myocardial infarction (AMI), and acute massive pulmonary embolism (PE). The high market share of thrombolytics can be attributed to the availability of approved AIS therapeutics like Activase, which has been on the market since 1987. This drug's widespread use is due to its ability to produce improved neurological outcomes in a significant number of AIS patients, leading to a high adoption rate among both physicians and patients. The inclusion criteria for administering IV treatment with Activase include a diagnosis of AIS causing neurological damage, minor neurological symptoms, onset of symptoms within 4.5 hours, no history of head trauma or prior AIS, no evidence of active bleeding, and normal blood pressure, among others. These factors contribute to the continued growth of the thrombolytics segment in the market during the forecast period.
Download a Sample of our comprehensive report today to discover how AI-driven innovations are reshaping competitive dynamics
Research Analysis
Acute Ischemic Stroke (AIS) is a medical condition characterized by the sudden interruption of blood supply to a part of the brain, leading to brain cell damage. Diagnostic tools for AIS include Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Carotid Ultrasound, Cerebral Angiography, Electrocardiography, Echocardiography, and various imaging techniques. Treatment options include Carotid Endarterectomy, Angioplasty, Endovascular Mechanical Thrombectomy, Thrombolytics such as alteplase and tenecteplase, Anticoagulants, Antiplatelets, Antihypertensives, Statins, and other Drug Classes. The route of administration for these therapeutics can be oral or parenteral. The distribution channel for these therapeutics includes Hospital pharmacies and Retail pharmacies. AIS is often caused by atherosclerosis plaques obstructing blood vessels.
Market Research Overview
Acute Ischemic Stroke (AIS) is a medical condition caused by a sudden disruption of blood flow to the brain due to the formation of blood clots in the arteries supplying the brain. AIS is a leading cause of long-term disability and the fifth leading cause of death worldwide. Neuroimaging techniques such as Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are essential in diagnosing AIS. Surgical interventions like Carotid Endarterectomy, Angioplasty, and Endovascular Mechanical Thrombectomy are used to remove the clots and restore blood flow. Medications like Tissue Plasminogen Activator (tPA), Anticoagulants, Antiplatelets, Antihypertensives, and Statins are used to treat AIS. Risk factors for AIS include atherosclerosis plaques, aging population, high blood pressure, diabetes, and obesity. The market for acute ischemic stroke therapeutics includes various drug classes such as Thrombolytics, Antiplatelets, Anticoagulants, and other Drug Classes. The route of administration for these drugs can be oral or parenteral, and they are distributed through hospital pharmacies, retail pharmacies, and online pharmacies. Neuroimaging technologies, including CT scans and MRI scans, play a crucial role in diagnosing AIS and monitoring its progression. The Defense Health Agency is one of the significant consumers of these therapeutics due to the high prevalence of stroke among military personnel. Some common diagnostic tools include Electrocardiography, Echocardiography, and Cerebral Angiography. The market for acute ischemic stroke therapeutics is expected to grow significantly due to the increasing aging population, rising prevalence of risk factors, and advancements in neuroimaging technologies and therapeutic interventions.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
- Type
- Thrombolytics
- Anticoagulants
- Antiplatelets
- Antihypertensives
- Others
- Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Geography
- North America
- Europe
- Asia
- Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/acute-ischemic-stroke-therapeutics-market-to-grow-by-usd-1-74-billion-from-2024-2028--driven-by-rising-cardiovascular-disease-prevalence-and-ai-driven-market-transformation---technavio-302278833.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/acute-ischemic-stroke-therapeutics-market-to-grow-by-usd-1-74-billion-from-2024-2028--driven-by-rising-cardiovascular-disease-prevalence-and-ai-driven-market-transformation---technavio-302278833.html
SOURCE Technavio

 Index Options
Index Options CME Group
CME Group Nasdaq
Nasdaq Cboe
Cboe TradingView
TradingView Wall Street Journal
Wall Street Journal