Natus introduces new FDA cleared Grass® MR Conditional / CT Cup Electrodes featuring longer lead lengths
MIDDLETON, Wis., Oct. 16, 2024 /PRNewswire/ -- Natus Medical Incorporated has announced the launch of Grass MR Conditional / CT Cup Electrodes featuring 28-percent longer lead lengths to promote easier application – the longest ever to be proven safe in 1.5T and 3T MR environments by receiving FDA clearance as MR conditional.
The FDA granted 501(k) U.S. Medical Device Clearance to the Grass MR Conditional / CT Cup electrodes, providing radiologists with the confidence that simple preparation will allow patients to safely and quickly move from EEG testing to MRI scans. The electrodes have also been tested to demonstrate low artifact in MRI environments.
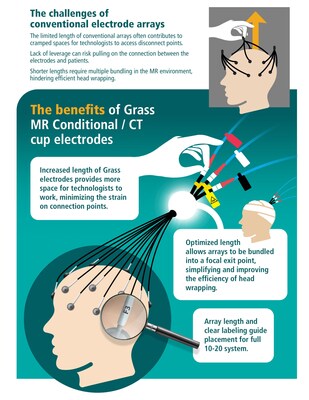
With deep-cup disposable single electrodes measuring 282mm in length and arrays extending to 270mm, the Grass MR Conditional / CT Cup electrodes enable easier application than the previous industry standard. The additional length gives technologists greater flexibility in accessing disconnect points before transport to MRI exams, minimizing strain on contacts with patients and ensuring easy reach for any 10-20 placement location.
"Our launch of the Grass MR Conditional / CT Cup Electrodes is the result of a journey that started by listening to the challenges that technologists, radiologists, and other healthcare professionals have experienced for decades while working with shorter MR-compatible electrode leads," said Chris Landon, Natus Neuro CEO. "Among the many benefits, longer leads provide more room for bundling and wrapping a patient's head with just one fixed exit point. Their ease of use also reduces the need for technologists to step in when a patient needs transferring for MR testing, especially during off hours, leading to savings in time and cost."
Grass MR Conditional / CT Cup electrodes are designed to provide a new standard in patient comfort, reducing the risk of skin breakdown by eliminating the need to disconnect and reconnect patients undergoing EEG monitoring in critical care environments. Because the detaching and reattaching of the portion of the cable that is MR unsafe is simple and intuitive for nurses and technologists, patients can expect a swift and safe transfer from busy ICUs to MRI exams and back. Limiting the interruptions to EEG recordings benefits the continuity of patient EEG monitoring and can lead to faster diagnoses.
Natus makes it easy to order MR Conditional / CT Cup electrodes and other supplies with a one-stop shopping experience. Streamlined order processing is available through the Natus Medical Store, available in select markets, or by contacting Natus' dedicated, knowledgeable supplies team.
The introduction of MR Conditional / CT Cup electrodes adds a new chapter to the Grass story and a Natus EEG heritage that reaches back to 1935 with the debut of the Grass Model I Electroencephalograph. The Grass Model I EEG established basic parameters to record EEG that remain established to this day. The Grass Model II EEG, introduced in 1938, was employed by the United States Armed Services during World War II when it was used in the selection of pilots and treatment of injured soldiers. Grass developed its first commercial electrodes in 1948, and has continued to raise the standard of quality, defining excellence in EEG signals.
Natus acquired the Grass Technologies Product Group from Astro-Med, Inc. in 2013.
ABOUT NATUS
Natus is trusted by healthcare providers around the globe as the solution source to screen, diagnose, and treat disorders affecting the brain, neural pathways, and sensory nervous systems. The best-in-class Natus solutions, including service, field support and education, enable clinicians to advance their standard of care, improving patient outcomes and quality of life. For more information on Natus, please visit www.natus.com/neuro.
CONTACT:
Lisa Schuler
Phone: +1 612 528-1332
Email: lisa.schuler@natus.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/natus-introduces-new-fda-cleared-grass-mr-conditional--ct-cup-electrodes-featuring-longer-lead-lengths-302278287.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/natus-introduces-new-fda-cleared-grass-mr-conditional--ct-cup-electrodes-featuring-longer-lead-lengths-302278287.html
SOURCE NATUS MEDICAL INCORPORATED

 Index Options
Index Options CME Group
CME Group Nasdaq
Nasdaq Cboe
Cboe TradingView
TradingView Wall Street Journal
Wall Street Journal